History of BNCT
Discovery of the neutron
The neutron was discovered by Professor James Chadwick of Cambridge University in 1932. In a famous episode, the husband-and-wife team of Frederic and Irene Joliot-Curie had noted a phenomenon that was tantamount to discovering the neutron when they found that an unknown form of radiation with an extremely high level of penetration was emitted when paraffin was irradiated with protons. However, they lost the honor of discovering the neutron and winning the Nobel Prize when they misinterpreted their results.
World’s first clinical research (U.S.)
A neutron source with a high fluence rate is essential if the approach is to be applied to cancer treatment. Consequently, any application of the idea had to wait for the emergence of nuclear reactors. Reactors at Brookhaven National Laboratory (BNL) and the Massachusetts Institute of Technology (MIT) were utilized in the first clinical research. Professors L.E. Farr and W.H. Sweet carried out clinical research targeting malignant brain tumors over the 10-year period beginning in 1951, but the clinical results were poor, with average survival times of less than six months. Concluding that the principal reasons for these outcomes were insufficient selectivity in the accumulation of boron compounds in tumors and the poor quality of the neutron beam, the researchers were forced to focus on resolving these difficulties. BNCT research in America was suspended and Japan subsequently picked up where the. Americans left off.
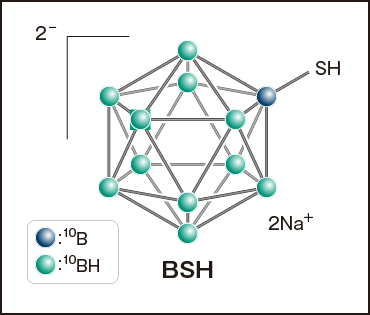
BSH : The first clinically effective boron compound
At the end of the 1950s, irradiation experiments using small animals as basic research began at the Japan Research Reactor No.1 (JRR-1) and the Hitachi Training Reactor (HTR). In 1968, a team led by Hiroshi Hatanaka began administering BNCT to patients with malignant brain tumors using a new boron compound (Na2B12H11SH, commonly known as BSH). The HTR and the Musashi Institute of Technology Reactor (MuITR), which was modified for medical use, were used as neutron sources, and a high-purity thermal neutron irradiation field developed at the heavy-water facility of the Kyoto University Research Reactor (KUR) was also used in 1974. Since the ability of the neutron beam (consisting of thermal neutrons) to penetrate deep tissue was limited, irradiation was performed during a craniotomy (in a process known as intraoperative irradiation). The compound BSH, of which each molecule has 12 10B atoms, is easy to use since it excels in its ability to transport 10B and offers high water solubility. Whereas BSH is prevented from penetrating brain tissue by the blood-brain barrier in normal brains, the failure of this barrier function allows it to penetrate, and accumulate in, malignant brain tumors, resulting in a large concentration difference. However, the compound’s properties are not such that cancer cells actively accumulate it. Although the results obtained by Hatanaka were favorable and suggested the efficacy of BNCT, he was unable to convince neurosurgeons and radiation oncologists of the usefulness of this treatment.(Also see "5. Boron Agents" section)
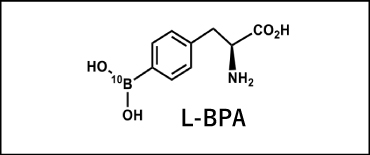
BPA: A boron compound that accelerated new developments in BNCT
Yutaka Mishima, formerly a professor of dermatology at Kobe University, conducted many years of research in an effort to treat malignant melanoma (skin cancer) exhibiting X-ray resistance with BNCT. He focused on L-paraBoronophenylalanine (L-BPA, referred to as “BPA” below) as an analog of tyrosine, which is a precursor of melanin, and conducted basic research to apply BPA in BNCT as melanoma-specific boron compound. As a result, there was a large difference in BPA concentration between malignant melanoma cells and normal cells. Through subsequent research using FBPA PET imaging, it was discovered that the compound accumulated not only in malignant melanoma, but also in various malignant tumors. It is thought that this fact is accounted for by the increased level of amino acid transport in malignant tumor cells, and today the effects of BPA-BNCT on numerous types of tumors are being studied. BPA was first used clinically in BNCT to treat a malignant melanoma in 1987 by Mishima and his team. The compound was first used in BNCT to treat a patient with recurrent malignant glioma in February 1994 using the KUR. This trial preceded a similar study at Brookhaven National Laboratory in the U.S. by about seven months. BPA differs significantly from BSH in that it is selectively absorbed by cancer cells, and it is fair to say that BNCT could first be called a technique for selective cancer cell treatment with the advent of BPA.Also see "5. Boron Agents" section)